The Investigation of Biomate PDL®
Connecting With the World
The Investigation of Biomate PDL® Designs & Surface Treatment Technique-Biomate Medical Devices Technology Co.
From the 1970s, since root-shaped oral implants were developed in the dental profession, the implant quality, technology and clinic procedure, even the success rate has been improving dramatically. Dental implants becomes a common practice in many doctor’s daily work. Though dental implants are reported carrying high success rates in most cases, there is still a poor healing problem in compromised alveolar ridges. There are a number of factors that might affect the healing, however, surface treatment and structural design along with the implant’s interaction with the surrounding tissues are still the crucial ones.
From the 1980s, the concept that the rough implant surfaces are better than the smooth ones had already been well established. But which rough surface is most appropriate for the bone healing is still debating. With the accumulation of the knowledge in bone healing, the cascade of bone healing around implants is complicated. It can be concluded in following procedures: right after the implant placement, fibrin is immediately synthesized and released from the injured blood vessels, and it then adheres to the surrounding tissues and the implant forms the provisional matrix. PMNs debridement and macrophages induction of fibroblasts to synthesize collagen follow. The collagen substitutes the fibrin and new blood vessels incorporates then form new connective tissue. The pericytes of the vessels surrounding the implant are inducted onto the implant surface through the collage scaffold, and the osteoclasts activated on the surrounding bone secreting BMP to induce the pericyte differentiating into osteoblasts. This leads to the new bone formation on the implant surface called de novo bone formation.
From the placement of implants to the formation of osteoblasts, almost every step is closely related to the microscopic structure of the implant surface, especially when fibrin adheres to the implant surface forming the provisional matrix and the osteoblast precursor cells migrate to the implant surface differentiating to osteoblasts. The adherence of fibrin is related to its hydrophilicity and hemocompactibility. For example: the SLActive implant of Straumann, the CA series of Osstem, and the “BIO-ACCEL” series of impladent from Lasak all emphasize on this point. These implants are further processed after the SLA treatment: SLActive is infiltrated in a saline solution, the CA series are infiltrated in calcium chloride solution, “BIO-ACCEL” series are further treated by hydroxidizing after its acidification in order to promote its hydrophilicity. In recent years, another type of laser surface treatment has gradually come to light. After laser surface treatment by high energy laser, its surface forms a layer of high energy oxidation. This also helps in its hydrophilicity and blood affinity. In Taiwan’s manufacturer Biomate, we can see both great blood affinity and adhesion of fibrin after its PDL surface treatment (figures 1A & B).
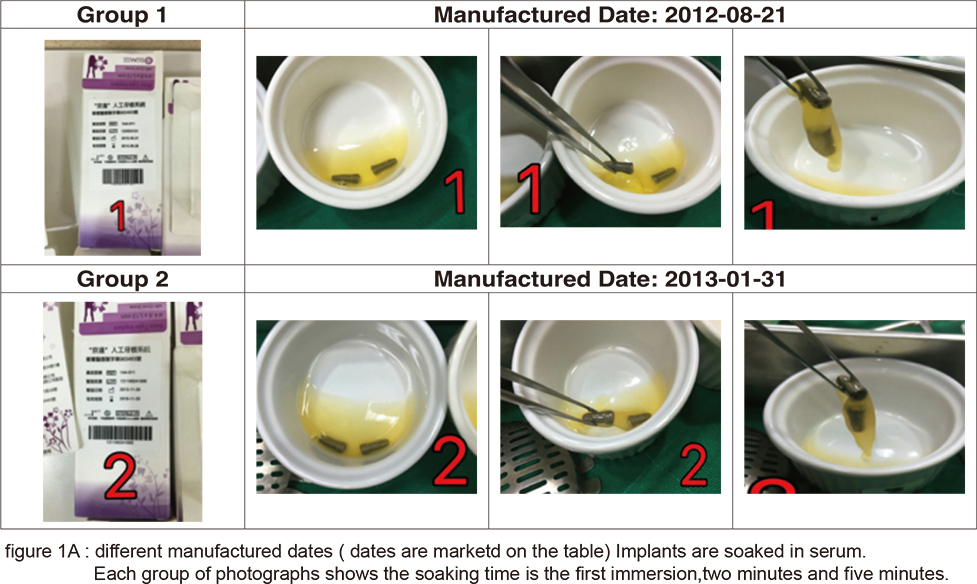
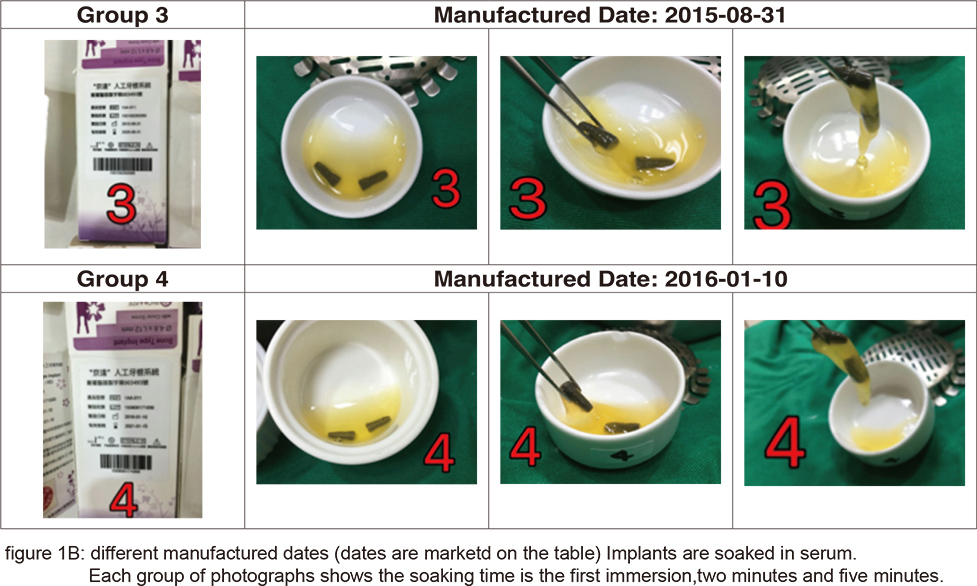
However, the most important mechanism in the bone healing is still in the proliferation and differentiation of osteoblasts procedure. In the development of rough implant surface, the SLA treatment is the most popular one. Though slight differences remain among manufacturers, they are nevertheless much the same. The biggest difference variates in quality control of the process of sandblasting, acid treatment neutralization and cleaning afterward. In contrast to SLA, laser treatment is a high cost and technique sensitive process. Thus, manufacturers have not yet reached a consensus on its protocol. The four methods derived from different manufacturers will be compared and discussed here.
Three of them are developed by renowned foreign manufacturers and the other one is developed by the domestic manufacturer mentioned above. The first type of implant is Leader Tixos from Italy, uses a high energy laser to sinter the titanium powder onto implant surface which is apart from the laser ablation technology of other manufacturers (figure 2 A). Though it possesses a high energy oxidized surface, its surface is obviously different from the micro-nano structures of other manufacturers.
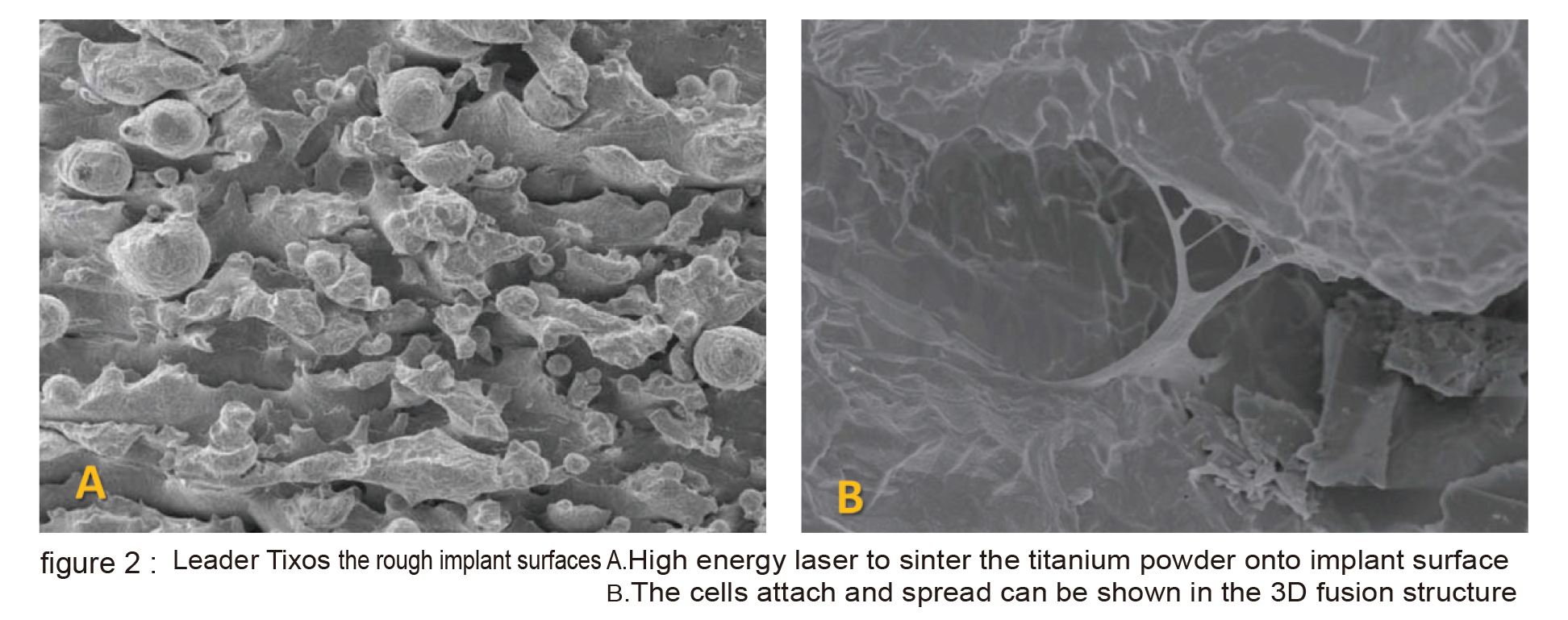
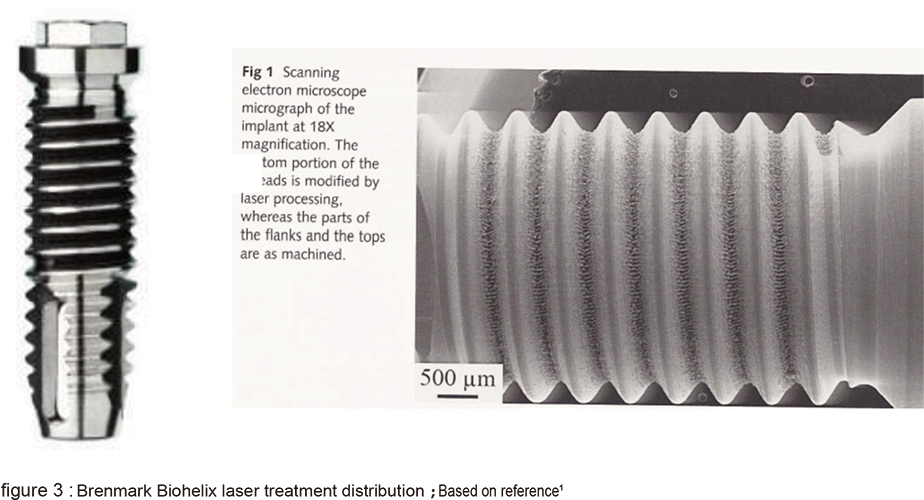
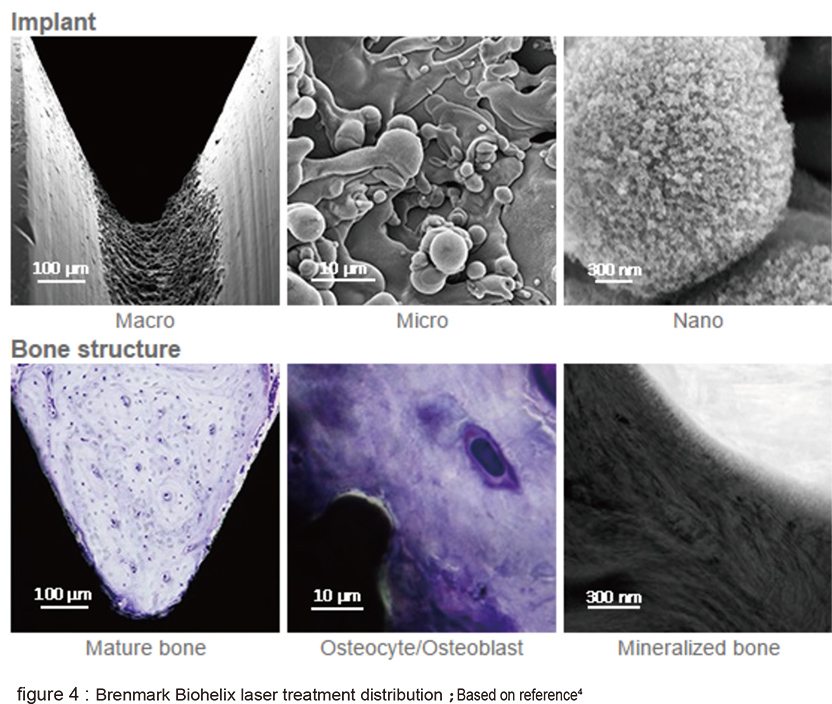
The cells attach and spread can be shown in the 3D fusion structure (figure 2 B). The second type is Branemark Biohelix implant; it is intended made to avoid the possible residue from SLA process by maintaining the whole implant in machined surface except for the middle middle portion of implnat which the valley portion of thread is laser modified (figure 3). The laser modified surface presents a nonlinear and obvious micro-nano structure (figure 4). The 5 year long clinical trial has provided promising outcome. The third type is Biohorizon Laser-Lok. It is totally different from Biohelix. The whole implant is SLA modified, except for the 3-4mm near its crest portion is laser modified.
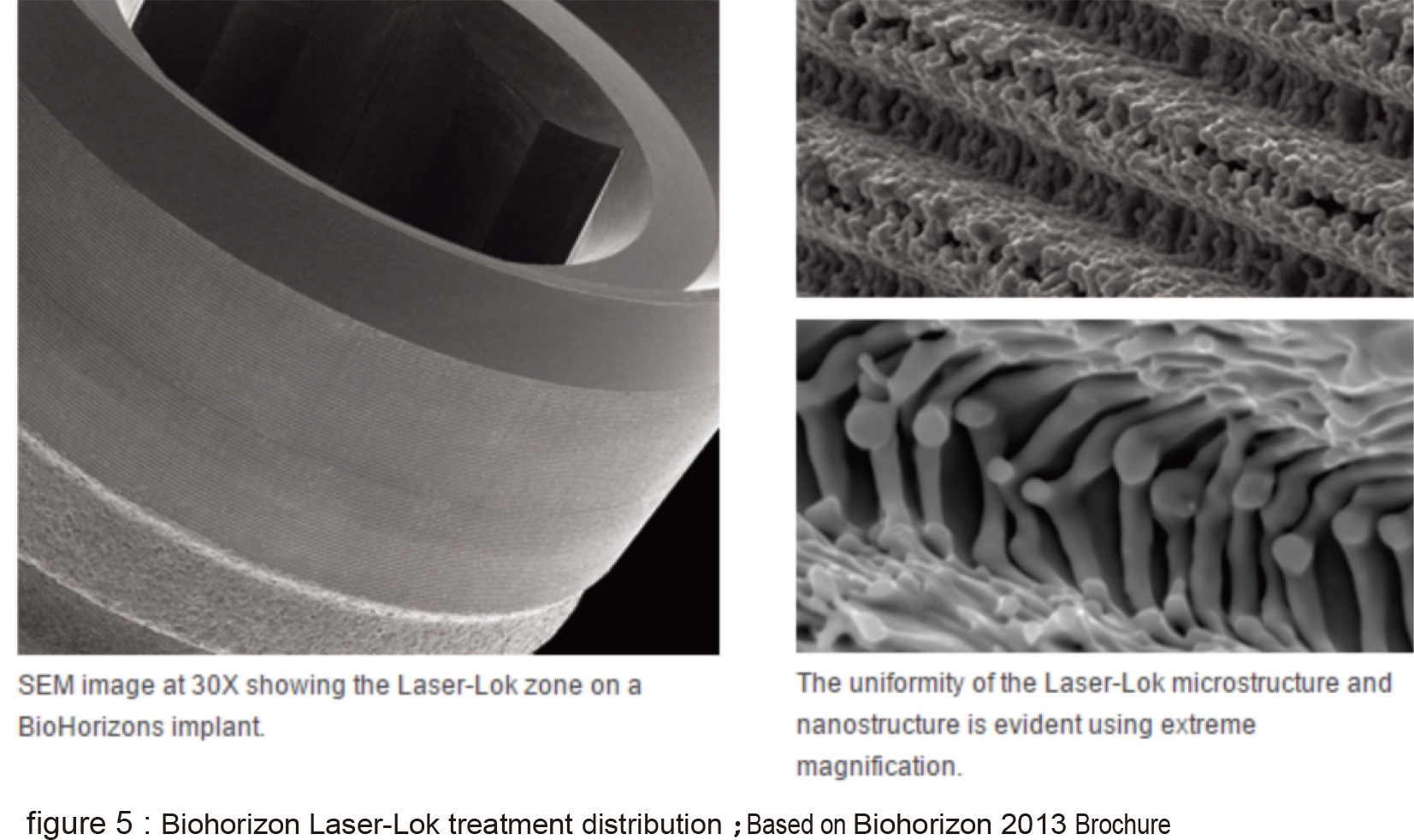
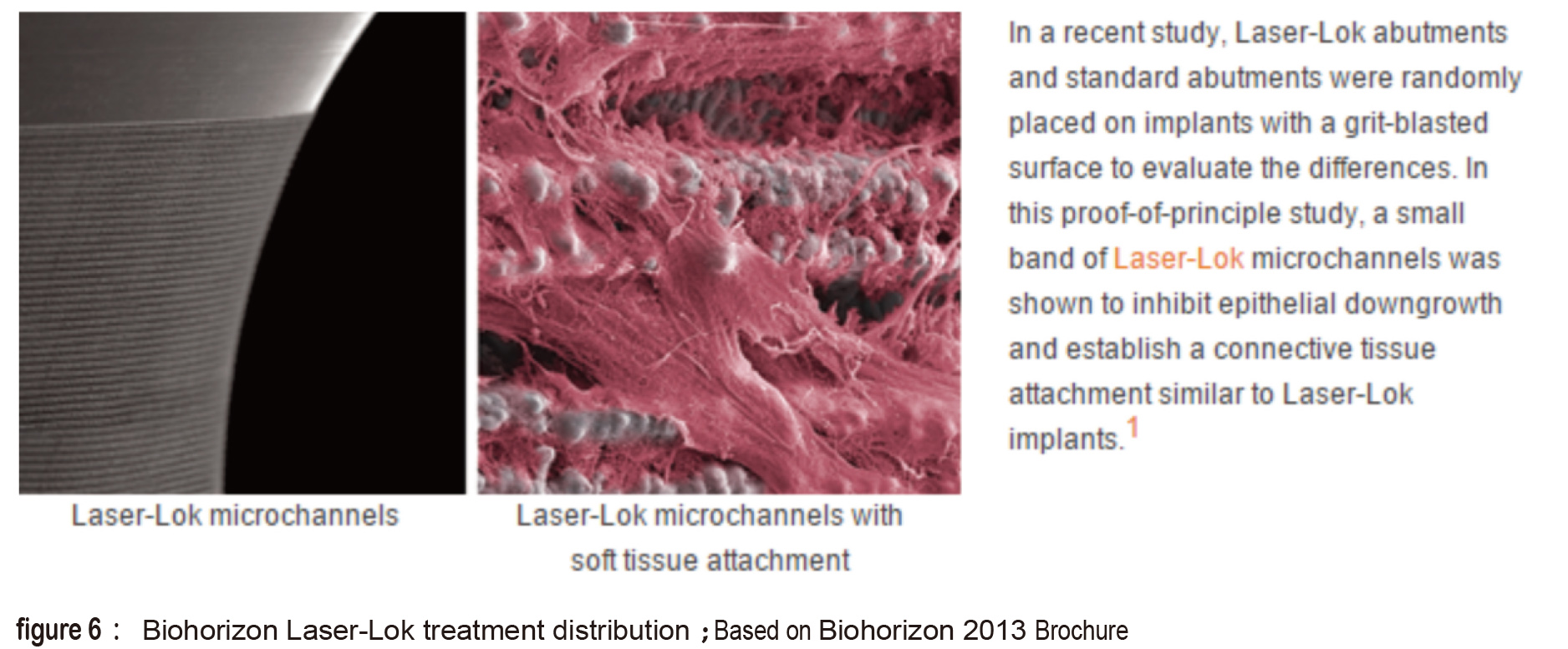
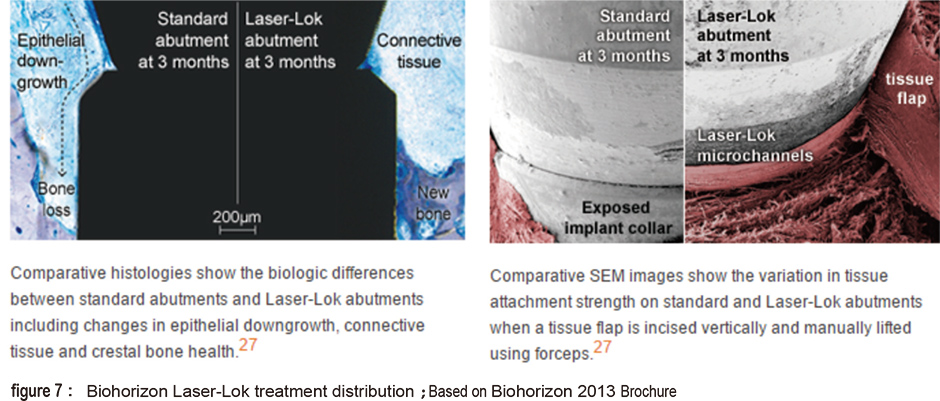
The laser treated surface forms special horizontal microchannels (figure 5) that theoretically helps the cells fast directional movement of fibroblasts and osteoblasts along the channel structure (figure 6). Theoratically, this can form an barrier near the crest portion which prevents epithelial cells and bacteria from invading which also prevents the possibility of pocket formation (figure 7). The fourth type is the domestic Biomate PDL™ implant. The original laser treatment concept is based from Taipei Medical University and the production is the collaboration between Industrial Technology Research Institute and Biomate Medical Devices Technology Co. (figure 8).
The entire surface, excluding the apex, is laser modified without SLA surface treatment or any other additives It is as “clean” as the Branemark Biohelix. In addition, due to the manufacturing process, the entire surface is covered with “vertical” (along with the long axis) directional microchannels (figure 8). The most particular feature is the pores or caves(figure 8) in the microchannels measuring at 10-15 micron in both width and depth. And the nano structures formed on the whole surface due to the laser treatment.
Designed with New Concept and New Technique,
it Possesses the Best Stability to Ensure Long-Term Efficiency.
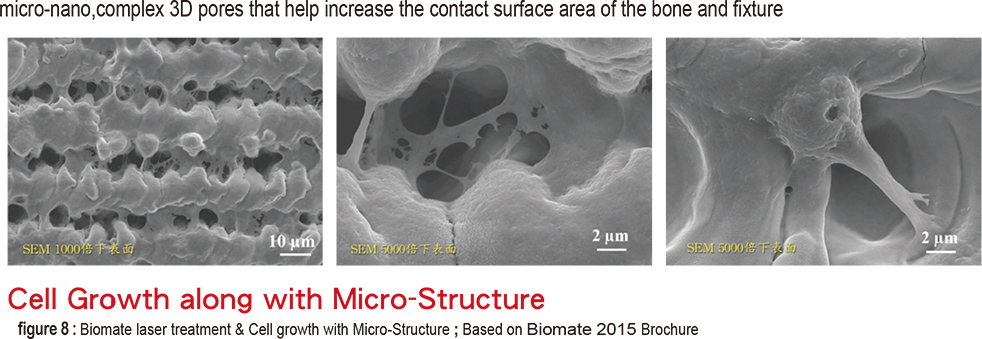
The pore structure is similar to a “cradle”, beneficial not only for the adhesion but also for 3 dimensional spreading of cells (figure 9 shows the linear arrangement of cells) and helping in activation (figure 9 c shows the growth phase of a, b and c; c is considerably faster). From the structure point of view, it should be more stable than the 2D adhesion and beneficial for cell differentiation. Moreover, the implant, as mentioned above, can form provisional matrixes quite easily. We can therefore anticipate that this particular implant will perform well in the bone healing process.
After the initial bone healing process, comes up the remodeling of mature bones. it is highly related to the occlusal loading. Screw thread is essential for transferring bite force to the bone surrounding the implant. Before further discussion about force distribution, one thing worth noticing first is that the high energy laser ablation can generate great stress. It may break easier compared to all other surface treatment techniques and most specifically near the crest portion of implant (figure 10).
This is highly stress concentrated portion and stress distribution is complicated. We need not only the solid implant structure but stable abutment design. Majority of current abutments are designed as hexagon. The most crucial point about this design is that there is a linkage effect among outer diameter of the implant, the implant wall thickness, hexagon of the abutment and central screw sizes. the interior would most certainly extrude with the size of the hexagon design and central screw.
However, the thickness of the implant outer wall must not be sacrificed. Usually, micro thread or shallow thread design is incorporated to increase the wall thickness and also helping distributing the stress. The double-layer hexagon design (figure 11; X’ive implant) can make the abutment more stable which allowing narrower central screw. Or shortening the extension of abutment along with a wider central screw is another option (figure 11 left; Implandent implant).
In the four lasers modified implants mentioned earlier, only two of them have been processed by laser ablation near the crest portion. We compare the differences between the two designs: Biohorizon does not have a screw thread design on the surface modified by laser ablation and this gives for a thicker outer wall; the abutment is design in one layer hexagon (figure 11; right). Biomate has a more complicated design in this area; its outer wall has a slightly tapered micro threaded design. The abutment adopts a 10 degree Morse taper combined one layer hexagon (figure 12). This design can decrease the stress around the ridge, strives for a reasonable thickness for the implant and stabilizes the abutment by minimizing the possible micro movement during occlusal loading. This design is suitable for the loosened bone cases, especially in maxilla.
In other parts of the implant, there are significant difference among manufacturers, so I will use the Biomate design as an example to explain the idea of the screw thread design: (figures 13&14)
- The lower part of the thread carries a larger angle of 25 degree allowing easier insertion of implant.
- The upper part carries a smaller angle of 13 degree preventing implant from dislodging.
- The space between the threads also forms an asymmetric trapezoid shape with a 0.3mm depth and 0.8mm apart. During the insertion of implant, the lower slope of this asymmetric trapezoid space (the upper part of the thread) squeezing the bone upward and compacting the bone into the space.
- The asymmetric trapezoid also benefits the laser processing on implant surface which providing good environment for optimal bone contact.
To sum up, the unique surface treatment and screw thread design allow Biomate implant to achieve osseointegration in both normal healthy and compromized bone structure.
From the three case reports shown below, we can see how Biomate’s PDL performs in poor quality bones:
Case report 1 (figure 15)
A 47 year old female patient used immediate implant placement on #16 with autologous growth factor (Concentrated Growth Factor; CGF) without bone substitute. From 6 month post-op follow up, new bone formation from the surface of the implant can clearly be seen in x-ray. After bone loading, the density was increasing over time, indicating that the implant surface treatment and screw thread design can help in bone healing from primary bone formation to bone remodeling efficiently.
Case report 2(figure 16)
A 61 year old male patient did not have sufficient ridge crest on #21. Implantation was performed combined with ridge augmentation using demineralized bone matrix mixed with autologous growth factors. 3 months later, a temporary crown was made followed the phase 2 surgery. From CT scans follow-up, it could be seen that from the day of surgery, the labial surface of the crest portion of implant was completely covered by bone substitute(1050108). However, one month after temporary crown loading, (1050504) there was obvious bone formation near the crest portion. The labial profile of cortical bone, was converted from concavity to convexity (compare 1050108 and 1050504, 1050610). This shows that a good implant crest portion design is beneficial for bone regeneration.
Case report 3(figure 17)
A 50 year old female patient had had #17 tooth extracted for 6 months; the ridge thickness was less than 1mm. The patient refused the lateral window surgery, thus, our initial plan was to performed the vertical ridge augmentation using piezo surgery with autologous growth factor mixing with bone substitute. And after bone healed, implant surgery could be followed. But it was noticed that it had enough resistance during the ultrasound bone expansion, thus, a 5.5x8mm implant was inserted. After three months of follow-up (1050928), an 8mm thick bone could be seen around the implant. The Biomate implant slightly tapered profile and screw thread design can condense bones during insertion and provide initial stableness. Even in patients in very poor maxilla bone conditions, a good healing process could be expected.
To develop a world renowned medical industry in Taiwan is no easy task. However, Biomate Medical Devices Technology Co. in collaboration with the Industrial Technology Research Institute, along with Biomate’s own screw thread profession, in coordination with Taipei Medical University Hospital, E-da Hospital, NCKU Hospital, Kaohsiung Veterans General Hospital, etc., have co-developed the PDL system. Because of its good healing results, it has earned an outstanding reputation and given a good performance in Southeast Asia, the Middle East and Russia. The effort from Taiwan’s industry deserves our appreciation and recognition.
References:
1. Ahmed M. Ballo, Omar Omar, Wei Xia and Anders Palmquist. Implant Dentistry - A Rapidly Evolving Practice Edited by Prof. Ilser Turkyilmaz. Chapter 2:Dental Implant Surfaces – Physicochemical Properties, Biological Performance, and Trends
2. Shu-Fen Chu, Min-Tsan Huang Keng-Liang Ou, Erwan Sugiatno,Han-Yi Cheng, Wen-Ta Chiu, Tsan-Hon Liou. : Enhanced biocompatible and hemocompatible nano/micro porous surface as a biological scaffold for functionalizational and biointegrated implants.2016. Journal of Alloys and Compounds
3. Rick C. Tsay. DMD, MD, Jennifer Vo. MS, Andrea Burke. BS, Sidney B. Eisig. DDS, Helen H. Lu, PhD. And Regina Landesberg, DMD. PhD: Differential Growth Factor Retention by Platelet-Rich Plasma Composites. J oral Maxillofac Surg 63:521-528, 2005
4. Mat Thomsson, Marco Esposito: A retrospective case series evaluating Branmark BioHelix implants placed in a specialist private practice following ‘conventional’ procedures. One-year results after placement. Eur J oral Implantol 2008;1(3):229-234
5. Thomsson M, Larsson Wexell C.: A 5-year retrospective case series evaluating Brånemark Integration BioHelix (™) dental implants placed in a private practice by a specialist. Acta Odontol Scand. 2013 Sep;71(5):1195-9.
6. Cary A. Shapoff, Brent Lahey, Perry A. Wasserlauf, David M. Kim.: Radiographic Analysis of Crestal Bone Levels Around Laser-Lok Collar Dental Implants. Int. J. Periodontics Restorative Dent 2010;30:129-137
7. Carlo Mangano, Francesco Mangano, Jamil A. Shibli, Lucia Tettamanti, Michele Figliuzzi,Susana d’Avila,Rachel L. Sammons,i and Adriano Piattelli: Prospective Evaluation of 2,549 Morse Taper Connection Implants:1- to 6-Year Data. J Periodontol. 2011 January;82(1):52-61
8. Vargas, P., Terriac, E., Lennon-Duménil, A. M., Piel, M. Study of Cell Migration in Microfabricated Channels. 2014.J. Vis. Exp. (84), .
9. Pradeep AR1, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB.: Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2012 Dec;83(12):1499-507
10. .Hi-Jin You and Seung-Kyu Han:Cell Therapy for Wound Healing, J Korean Med Sci. 2014 Mar; 29(3): 311–319.
11. Carin Hallgren, Henrik Reimers , Dinko Chakarov , Julie Gold , Ann Wennerberg: An in vivo study of bone response to implants topographically modified by laser micromachining. Biomaterials 24 (2003) 701–710
12. S. Zimmermann , U. Specht , L. Spieß , H. Romanus , S. Krischok , M. Himmerlich , J. Ihde: Improved adhesion at titanium surfaces via laser-induced surface oxidation and roughening. Materials Science & Engineering A 558 (2012) 755–760
13. Andreas Heinrich & Katrin Dengler & Timo Koerner & Cornelia Haczek & Herbert Deppe & Bernd Stritzker: Laser-modified titanium implants for improved cell adhesion. Lasers Med Sci (2008) 23:55–58
14. Davide Berardi, Simona de Benedittis, Andrea Scoccia, Giorgio Perfetti, Pio Conti: New laser-treated implant surfaces: A histologic and histomorphometric pilot study in rabbits. Clin Invest Med 2011; 34 (4): E202-E210
15. Mingdeng Rong, Lei Zhou, Zehong Gou, Andi Zhu, Dongfeng Zhou: The early osseointegration of the laser-treated and acid-etched dental implants surface: an experimental study in rabbits. J Mater Sci: Mater Med (2009) 20:1721–1728
16. Sung-Am Cho, Sang-Kyoo Jung: A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials 24 (2003) 4859–4863
17. Enhanced biocompatible and hemocompatible nano/micro porous surface as a biological scaffold for functionalizational and biointegrated implants Shu-Fen Chu, Min-Tsan Huang Keng-Liang Ou, Erwan Sugiatno,d, Han-Yi Cheng,d, Wen-Ta Chiu,Tsan-Hon Liou. Journal of Alloys and Compounds (2016 accepted)
18. Ming-Hung Tsai, Chiung-Fang Haung, Shih-Shiun Shyu, Yen-Ru Chou, Ming-Hong Lin, Pei-Wen Peng, Keng-Liang Ou, Chih-Hua Yu.: Variation on the rapidly solidified recast layer of titanium. Materials Characterization 106 (2015) 463–469

